Local search and local SEO: the ultimate guide

There are two types of search: the regular organic search results and the local ones. Whenever you search for something that might have a local intent, Google will tailor its results around that query. That part is called local search, and when you try to rank your business in the local search results, you are doing local SEO. In this ultimate guide, we’ll explain the ins and out of both and help you with your local search strategy.
Table of contents
- What are local search and local SEO?
- An important distinction: organic vs. place
- Your first stop: Google Business Profile
- Website optimization for local SEO
- The importance of relevance
- The changing place of your website
- Inbound links are important
- Citations and online mentions
- Reviews: more important by the day
- Behavioral signals
- Knowledge Panel interactions
- In-store visits
- Offline transactions
- Social media is nice to have
- Local SEO conclusion
What are local search and local SEO?
Local search refers to all the activity in search engines that results in a local-oriented result. In the context of Google Search, local search engine optimization (local SEO) aims to improve the visibility of a website or business in Google’s Local Pack, Google Maps, and other local search results.
Local SEO uses various techniques, including creating and optimizing Google Business Profile listings, incorporating location-specific keywords into website content and meta tags, and obtaining positive customer reviews to improve visibility in local search. Local search optimization is an important aspect of improving a website’s visibility in search engines for users searching for businesses or services in a specific area.
An important distinction: organic vs. place
You might say it’s all Google, so how different could the local results be compared to the regular, organic ones? And it’s true, at its core, Google has always tried to provide searchers with the ‘best’ result for a given query. But the ‘best result’ depends on the context of the query. The type of search and the location of the person searching provide Google with two vital pieces of context.
Webpage-related results
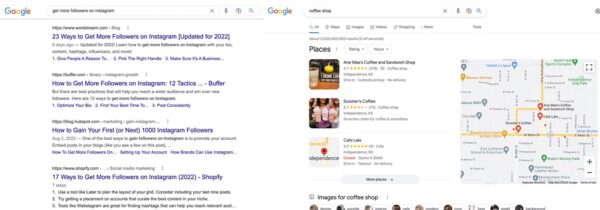
Consider a search like get more followers on Instagram. No matter where you’re performing that search — mobile or desktop, home or on the go, in India or Iceland — you’re looking for an answer anywhere in the world. You’ll primarily find the answers on web pages in the ten blue links, with products, featured snippets, case studies, or articles about how to do so.
Place-related results
With a search like coffee shop, Google has high confidence that you’re looking for a place to grab a coffee now. You probably want a place close to you, no matter where you’re searching. Sure, we could browse a magazine article about the best coffee shops in this city or look at a complete list of coffee shops on a directory page. But it’s much more helpful for Google to return a list of places than other websites about places.
Differences
Google’s webpage-related results for Instagram followers and its place-related results for coffee shops are generated by different algorithms. Searches with specific questions like How do I make chimichurri? are likely to trigger a different kind of result in the form of rich results. But that’s a topic for another day.
As a local business, you will face fierce competition in webpage-related results. If you offer services to help get more Instagram followers, you’ll have to compete with every other provider of this service to get your website ranked.
But in the second instance, when Google detects a search with local intent, you’re only competing with other coffee shops near you. Note above; we didn’t specify our city; Google inferred it. And even though Starbucks has coffee shops in just about every town and city in the world, it’s harder for them to stand out against local brands in these place-based results. And these results are also featured in Google Maps, in-car navigation devices, Google Home/Assistant searches, and many other media.
More place-based results
Over the last few years, Google has shown more place-based results for local queries and fewer webpage results. Even the webpage results that appear beneath these place results on a local intent search have been infused with local business websites.
Regardless of the medium (desktop, mobile, or voice) and irrespective of the type of result (webpage or place-related), Google remains a significant source of customers for many local businesses. So it’s critical to put your best foot forward to attract those customers in both algorithms. You can use (local) SEO and a solid local search strategy to do so.
Your first stop: Google Business Profile
Having a strong online presence is essential for any local business. With more people than ever searching online for products and services, the first step on the way to success for your local business is to create a Google Business Profile.
Google Business Profile — previously known as Google My Business — is a free tool that allows local businesses to promote their products and services and provide customers with critical information. Having a Google Business Profile has numerous benefits and is a must for any business that wants to stay competitive in the digital world.
Read our guide on Google Business Profile and how to optimize it.
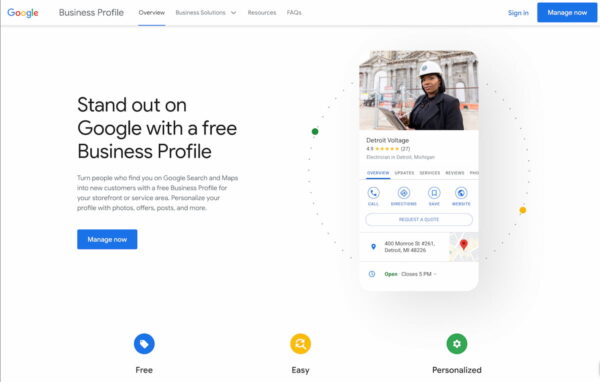
Increase visibility on Google Maps
One of the most important reasons to have a Google Business Profile is to increase your visibility on Google Maps. Being listed on Google Maps allows your local business to be found more easily by customers searching for your products and services. It helps customers find your business quickly and easily, including a map of your business location, contact information, opening hours, and more. This is a great way to boost local visibility and get more customers in the door.
Grow local customer base
Growing a local customer base is one of the key reasons why every local business should use Google Business Profile. By creating a profile, your business will appear in search results and Google Maps, allowing potential customers to find and contact your business. Furthermore, using the various features, you can promote your local business to potential customers by adding photos and other important business information. Doing so will help potential customers find you while making a positive impression on them.
Gain insights into customer activity
Google Business Profile helps you leverage insights into customers’ activities. Businesses can use analytics to identify customer trends, preferences, and behaviors. You can use this data to create targeted marketing campaigns and outreach initiatives. Businesses can also use the metrics to track the performance of their campaigns and make improvements based on customer feedback. With this data, companies can arm themselves with the knowledge to effectively serve their customers and increase sales.
Manage your business information
Having up-to-date and complete information about your business on your Google listing can significantly affect how customers find and interact with your business online. Keeping information such as your business hours, contact information, and services offered current and accurate ensures that your customers have the best possible first experience. Additionally, customers can leave reviews on your listing, which allows you to manage your online reputation better and allows potential customers to learn more about your business before they visit.
Connect with customers and manage reviews
Google Business Profile allows local businesses to connect with their customers as they can leave reviews about their experiences. By managing reviews, companies can demonstrate to potential customers the quality of their products and services. This is a great way to build customer trust and loyalty, and it can help generate leads.
A Google Business Profile is essential for any local business looking to do well in local search. By optimizing and maintaining an up-to-date profile, companies can ensure they are visible to potential customers and have the most up-to-date information about their business easily accessible to customers.
We have an extensive guide on making the most of Google Business Profile for your local SEO efforts.
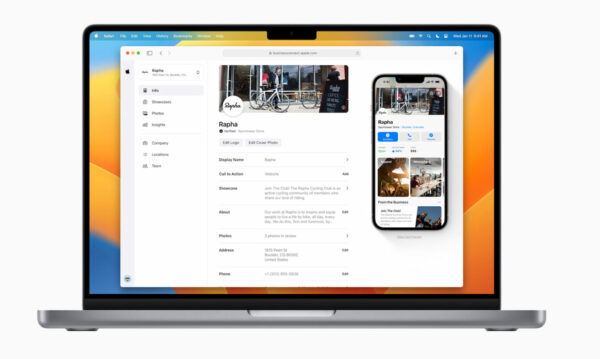
Apple Business Connect
In January 2023, Apple launched a similar platform under the Apple Business Connect moniker. Business owners will now have the ability to self-manage their information on the platform, including crucial details such as business hours, location, photos, logos, and special deals. To facilitate this, the company launched a website named Apple Business Connect, which enables businesses to oversee their presence across Apple’s 1.5 billion devices from one central location.
Be sure to check it out!
Website optimization for local SEO
Your website is one of your most important pieces of digital real estate and one of the fundamental components of a successful local marketing stack. It’s a crucial communication vehicle from you to your customers. Regardless of changing consumer search and social media behavior, it will remain a place consumers visit. It’s where people get more information about you and connect with your business.
Your website is the ranking factor over which you have complete control. This makes it an ideal asset to begin your local marketing campaigns powered by your local search strategy. We’ll review some important website optimization criteria, also known as on-site or on-page SEO. Thanks to local SEO, improving your performance across these criteria will help you rank better for local searches and attract more customers.
Crawlability
Google has built a giant database of hundreds of trillions of web pages which its algorithm analyzes and ranks. It sends scores of robots or spiders visiting page after page. They follow the links on each page to see where they lead. This is called crawling.
Technical issues
You want to ensure that Google’s spiders crawl your website and store its contents in its database. The quickest way to assess your website’s crawlability for major hurdles is to enter this search at Google: [site:yourdomain.com]. For example:
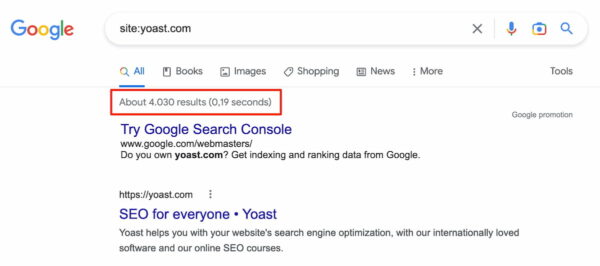
Before you browse the results list, look at the number that Google returns and see whether it’s more or less accurate. For example, if you have a 5-page site and Google returns 1000 pages or a 1000-page site and Google only returns five pages, you have a major technical issue with your site. You may want to dive into that with the Yoast SEO plugin or even bring in outside assistance.
You should also register your website with Google Search Console for additional technical advice and testing tools. Here’s a guide to get you started with Google Search Console.
Site structure
Site structure concerns the arrangement of your website’s functional and visual aspects. Essentially it’s the hierarchy of pages within your site and the content within each page. Regarding local search and SEO, there are a couple of essential best practices for your site architecture.
First, place your basic contact information in the header and footer of your website. You want to make it easy for customers who land on your website to contact you or complete a transaction — no matter what page they enter first.
A dedicated “Contact us,” or an “About us” page with more detailed information about your business is also a good idea. Ensure you link to this page from your homepage and, ideally, from your primary navigation menu.

Contact page content
Your contact page should contain the same information you submitted to Google Business Profile (address, phone number, and hours). It should also contain an email address or contact form for customers who prefer email to voice calls. If you collect customer reviews and testimonials, this is a good page to include at least a handful. Be sure to give it the proper contactPage structured data in the advanced schema options of Yoast SEO.
If you’re a traditional brick-and-mortar business, you should include written driving directions from population centers near you. These driving directions help prospective customers and Google identify the markets you serve. Include an embedded Google Map, too, as Google may track clicks for driving directions as a ranking factor.
If you’re a Service Area Business, your contact page should mention the significant surrounding towns and cities your business serves. You might consider building a unique page for these substantial towns and cities. Link to them from your contact page and fill them with case studies and testimonials from customers in those markets.
Advice for businesses with multiple locations
If your business operates in more than one physical location, creating a unique page for each is essential. Including a unique page for each location helps your customers (and Google) avoid conflating contact information between them. It’s also best to expand your local ranking potential to multiple cities. These pages also allow you to go more in-depth with localized content for each location, making it a good option for local SEO.
If you operate a handful of locations, link to the contact page for each one from the footer of each page of your website. Connect to a store locator page from your primary navigation or another utility menu if you use more than a handful.
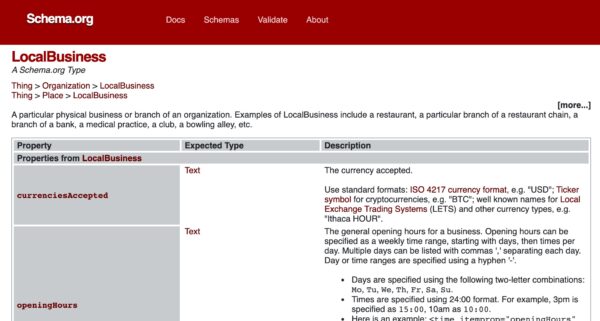
Don’t forget to add Schema.org structured data
Schema.org is a code protocol developed jointly by the world’s top search engines. It was created to make it easier for companies to structure the data they present on their websites. One of the most widely-used schemas is for business contact information.
Marking up your most important information in structured data is like handing Google a business card. Google’s pretty smart, but rather than leaving to chance that it will be able to crawl your contact info, why not do everything you can to guarantee it? Adding structured data will not improve your rankings, but it can give your organic results some extra visual impact, increasing the chances that customers will click on your result.
There are various schemas for LocalBusiness, with more added all the time, including LegalService, AutomotiveBusiness, and more.
Crawlability made easy: Yoast SEO plugins
The Yoast Local SEO plugin takes care of what we mention in this article on local search. You’ll have to add the proper pages to your WordPress website and link them appropriately from your menus. But the plugin handles most of the technical details required for your contact page, and we highly recommend it. In addition, it also comes with a load of LocalBusiness structured data options so you can easily pick your business from a list.
Yoast SEO Premium gives you more control over what search engines crawl on your site. You can use the crawl settings to guide them away from URLs, assets, and scripts that are unhelpful or add unnecessary overhead.
Mobile SEO
For the last couple of years, it has been all about mobile. Mobile will remain a significant factor in the coming years. Therefore, you should make your website faster and easier for mobile visitors to use. This process is called mobile SEO.
Test your site’s mobile friendliness
Google provides this easy-to-use free tool to test how friendly your website is for mobile visitors. It warns you about any significant suboptimal features and renders a screenshot of how your site appears for most mobile visitors.
Improve the mobile user experience
Google also provides a detailed guide on improving your website’s user experience for mobile visitors. Critical aspects of user experience to keep in mind:
- Does the width of your website automatically adjust to the screen size (“viewport”) of the visitor’s device?
- Does text automatically resize for mobile visitors, so they don’t have to pinch and scroll to read it?
- Are your calls to action and other buttons large enough for people to click with their fingers and thumbs?
These adjustments for the mobile visitor comprise what’s known as responsive behavior. If your WordPress website is not yet responsive, it’s time to upgrade your theme to one that is.
Make your site faster
One of the most significant website improvements you can make is to get your site to load faster. We’ve all been frustrated by sites that load slowly or won’t load on slower data connections. Sites that load quickly help build positive engagement with your business, and some evidence suggests that loading time and engagement with your content improve your rankings.
Conveniently, Google also provides a free tool to assess how quickly your site loads relative to others. This one is an extremely tough grader, though! Nonetheless, if you want to supercharge your website speed, Google provides advice on how to do it in the Opportunities section of this tool. But one of the easiest and most effective ways of speeding up your site is by upgrading your hosting plan.
The importance of relevance
Thus far, we’ve focused mainly on the technical aspects of your website. But if your technically-optimized website doesn’t feature relevant, high-quality content, you will rank poorly — and attract very few customers. From a content standpoint, the goal of your website is to communicate to both Google and users precisely what products or services you offer and where you offer them.
What keywords or keyphrases to target
At the risk of stating the obvious: you want to be relevant for topics, keywords, and phrases your customers are searching for. This typically means using generic layperson’s terms to describe your products and services instead of industry jargon (unless you’re in a very niche business-to-business industry). An example from the medical field would be to use an “ear, nose, and throat doctor” instead of an “otolaryngologist.”
Keyword research is an important part of SEO, and that also goes for local SEO. Here are a couple of easy sources for good keywords to target:
- Pay attention to the language customers use in their phone calls with you (or your staff) and emails and contact forms.
- Pay attention to the category terms that Google Business Profile returns when you type related keywords.
- Perform a search for each of the terms above and scroll to the bottom of the results page. Google will list terms related to the one you searched for, front and center.
Build a master list of these terms and match them up with local landing pages on your website, one keyword to one page. It’s entirely likely each page will rank for far more terms than the keyword you target. But it’s good to keep your pages focused on a small handful of terms. Below is a small example of how you could do this:
| Page | Parent | Target keyphrase | Title tag |
| Testimonials | Home | Best furniture stores in Newark | Moe’s furniture: Rated one of the best furniture stores in Newark |
| Vintage | Sofa styles | Vintage sofas in Newark | Vintage sofas Newark | Pick the vintage sofa of your dreams |
| Modern | Sofa styles | Modern sofas Newark | Newark! Come find the perfect modern sofa at Moe’s furniture |
| Scandinavian | Sofa styles | Scandinavian design sofa Newark | Scandinavian sofa designs in Newark | Moe’s furniture |
In addition to discussing your products or services, you should include your city, state, or metropolitan area as part of these keyphrases. Google has gotten better at detecting the area a local business website serves — particularly for websites that use structured data. But it’s still a good practice to sprinkle these geographic keywords liberally within your website.
Where to place your keywords
Your title tags are the most important places to put your keywords. Remember, though, that Google might rewrite your titles when it thinks it can do a better job. Note that title tags and the page or post titles you enter in WordPress are different. Title tags are the SEO titles in Yoast SEO.
Perform the site:yourdomain.com search we mentioned earlier to see your existing title tags. The blue link text associated with each page in these results is the page’s title tag.
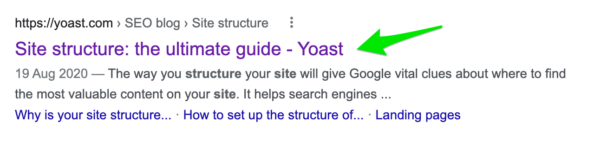
Yoast SEO helps you edit your SEO title tags. Pull up your target keywords and add them to the corresponding pages.
Take some time in crafting each title, though. Don’t just stuff your keywords in and then tack on your city, state, region, or county at the end. Remember that in addition to conveying to Google the terms for which you want your business to be relevant, these are the phrases your prospective customers will see when searching. So make these titles enticing for visitors as well as keyword-focused. If you don’t do a good job, Google will rewrite them.
For example, which title tag would you be more likely to click?
Option 1:
Car Insurance Agent - Luxury Car Insurance Agent - Car Insurance Agency - Newark, New Jersey
Option 2:
Newark’s Top Locally-Owned Car Insurance Agency since 1954: Smith Insurance
We’d undoubtedly choose option two, and most of your customers would do the same.
It’s also a best practice to include your target keywords in your WordPress page/post titles and other headlines. Nevertheless, it’s far more important to write these for your visitors than it is to write them for Google.
The final place to use your keywords is within the links you use on your website, also known as anchor text. For example, instead of saying click here — which you should never use anyway –, you might say contact our insurance agency to help Google gain a little more context about what services your contact page is relevant for.
The changing place of your website
We’re moving into a world with more place-based (mobile and voice) results and fewer website-based (desktop) results. Increasingly, Google is trying to extract as much structured information as possible from your website and place it in the Knowledge Graph Panel it constructs with the data from Google Business Profile.
This shift is why crawlability is such an important part of local SEO. Your website must give Google a strong sense of what you do and where you do it, but it’s even more important that Google can crawl that information, assimilate it, and present it in a structured format.
As a result, tactics like LocalBusiness Schema.org markup and tools like the Yoast Local SEO plugin that help structure information about your business are becoming that much more important. Your content is still critical, but start thinking of your website primarily as a data source for the Knowledge Graph and as a customer destination secondarily.
Inbound links are important
Since the ascent of Google as the world’s top search engine, links have been the primary concern of most SEO practitioners. The seminal idea behind Google’s ranking technology makes it clear that inbound links are the primary vehicle by which Google discovers new online pages and websites. They’re one of the ways Google assesses the credibility of a given website — although the importance of links is waning.
Google’s emphasis on links is the most significant overlap between its organic and local ranking algorithms. According to the experts of the Local Search Ranking Factors survey, links make up an essential piece of the pie in localized organic results. They’re a competitive difference-maker across all types of local results.
Local businesses can’t be thoroughly evaluated based on links for reasons you’ll see further on. But there’s no question that a solid inbound link profile — links pointing from other websites to yours — positively impacts how well your business ranks. Link building should be part of your local SEO efforts.
Why links in the first place?
You’re probably thinking, “hey, I want to rank #1, just tell me what to do!” But understanding why Google values links so highly can help you assess the strength or weakness of your link profile. This can help you determine your link acquisition strategy.
Googlebot crawls the Internet by following one link after another. They discover new pages and websites as part of that crawl and store the content of each of those pages in a giant database.
In addition to storing the content of each page, Google also stores how its crawlers arrived on the page. In other words, it remembers the pages and websites linking to it. A link from one site to another is like a vote or endorsement for the credibility of the second website.
Sites with the most endorsements rank better than those with few or no endorsements. Especially links from websites that are heavily endorsed themselves improve your ranking. You need endorsements to get elected and links to rank well.
Link attributes
Topical context
While Google’s algorithm over the years has been incredibly vulnerable to abuse by spammers, increasingly, it’s taking into account the context in which a link appears. Google essentially devalues links that appear on completely unrelated websites. For example, a personal injury lawyer receives a link from a Russian real estate forum. Increasingly these kinds of links put you in jeopardy of a Google penalty.
Conversely, links you acquire or earn that are likely to refer you to actual customers are increasingly the ones that Google values. For example, a personal injury lawyer receives a link from a neighboring chiropractor’s website.
Page/domain authority
The source of a link matters a great deal to how much weight it carries in Google’s algorithm. Links from pages and websites that are heavily linked to — such as BBC.com or WashingtonPost.com — will benefit the linked site much more than a link from a hobbyist blog or tiny startup.
Anchor text
Anchor text is the words that make up the link itself. The link’s text helps provide Google additional context about the topic of the linked page, i.e., what keywords that page should rank for. So links that contain keywords related to what you sell or where you’re located — and even links for your brand name — will help you rank. They’ll help you more than links using generic terms like “click here” or “read more.”
But you don’t have control over what text people use on other websites. In general, it’s not the best use of time to influence what anchor text others are using. It’s just a ranking factor to be aware of.
Assessing your existing link profile
Many SEO tools — like Semrush, Moz, and Ahrefs — help you analyze your existing link profile, which provides all the information an average local business needs.
See if you can find one which lists the authority of the domains linking to you, often described as page or domain authority. The number of referring domains is the best heuristic for most local businesses regarding their strong link profile. Explore the list of the sites sending links to you. Are there apparent sites not on that list that should relate to you? Consider reaching out to them to tell them how much a link would help your business.
Links that help your local search strategy
Google pretends that great content and websites will naturally acquire links. But for 99.999% of businesses, that’s terrible advice. The old question, “If a tree falls in a forest and no one’s there to hear it, does it make a sound?” applies to content and links. Does it acquire links if you produce great content, but no one’s there to see it? The answer is no. Businesses need to be proactive about reaching links, which makes building links an important part of your local search strategy.
Over the years, many local businesses have fallen victim to scam artists selling hundreds of links. Or have otherwise been too aggressive about acquiring links. The reality is that, for many businesses, 10-20 high-quality links will lead to top rankings in short order – sustainable rankings that will last for years. Take the time to earn these high-quality links, and don’t pursue those over-aggressive tactics.
Industry-relevant links
Industry-relevant links are often the most accessible links for small business owners to acquire. Many involve asking your existing contacts at companies or organizations with whom you do business.
Local business and neighborhood associations
Are you a member of your local chamber of commerce, business association, or neighborhood association? Most groups like these operate a member directory, and you want to ensure that the directory is online, visible to the public, and Google’s spiders. If the websites of these groups are not showing up in your backlink profile, bring up the issue with the director or marketing manager of these associations and ask them to create a webpage that links to each member.
Regional/national certification boards and industry organizations
Depending on your industry, you may also be licensed by, or participate in, a regional or national organization. Don’t just display your certification on your website. Link to your business’s online profile on the websites of these certifying boards and industry organizations. This increases your business’s credibility with potential customers and helps Google’s spiders discover and crawl your profile on these highly-trusted sites.
Distributors (directories or announcements)
For retailers, think about the products that you sell in-store. Are you unique or one of the few stores in your local market that carries a particular product? If so, consider asking the manufacturer or distributor of that product for a link from their website, preferably from a “where to buy” directory. At the very least, these companies should partner with you on a press release – containing a link to your website. For example, to announce to their customers — and Google — where people can buy their product in your area.
Vendors (testimonials)
Are there particular vendors from whom you purchase many goods or services? Ask them if you can contribute a testimonial to their website, and if they really appreciate your business, that testimonial will contain a link back to your site.
Interviews and guest columns
Getting featured in a trade publication is a great driver of business – especially referral business – and can provide a vital link to your website. These links are a little more challenging to acquire as they require building a relationship with authors or influencers in your industry.
Locally-relevant links
Charities or schools to which you’ve donated money, goods, or volunteered.
Many of you are likely involved in local charities on non-profit organizations. These links are highly valued by Google, as charities tend to be trusted institutions offline and online. You want to make sure your involvement is acknowledged online. They’ll give you a link from their website if they want to thank you for your involvement.
Groups for whom you host events at your physical location
Hosting events for outside groups is one of the lowest-cost, lowest-work link-building initiatives you can undertake. Chances are good that the business or group hosting the event at your company will link to your website’s contact/directions page when they post their invitation online. Someone else is building your link for you – and who knows – some of the attendees may even turn into customers!
Complementary businesses
You probably have colleagues in related industries to whom you refer business and from whom you’re referred regularly. Make sure these referral relationships are represented online in the form of links. That way, Google knows that your companies vouch for each other just as you do offline.
Interviews and guest columns
Local publications like newspapers and alternative weeklies or monthlies are terrific places to get your business featured. And the chances may be better, especially in smaller towns or tightly-knit neighborhoods, that a friend of a friend works at one of these companies.
The future of links and rankings
SEO professionals have been predicting the demise of links for many years. But there’s little evidence to support this trend, even though Google’s John Mueller recently hinted at a future where they might rely less on links. Google has gotten better at penalizing low-quality links through various algorithm updates. Still, if anything, high-quality links have been that much harder to come by and even more valuable to their recipients.
Citations and online mentions
In 2005, the internet was a very different place. MySpace, not Facebook, was all the rage, and Twitter, Pinterest, and Instagram weren’t even close to launching. There was no iPhone, and there was no Android. In a nutshell, the world was far less digital. When you searched at Google, it returned ten blue links of webpage results. Inbound links largely determined the authority of those web pages.
But the launch of Google Maps in early 2005 and the subsequent release of the 10-pack in May 2007 introduced something entirely different. Google Maps and the 10-pack ranked business listings, not websites, required a completely different algorithm – an algorithm that remains distinct today.
Google has changed a lot over the years, but the underlying foundation of that Maps/10-pack algorithm still seems to be in place today in the Maps/3-pack interface that has succeeded it.
What’s a citation?
A citation references your name, address, or phone number online. While inbound links were the dominant ranking factor for the ten blue links results, Google’s listing-based results couldn’t rely primarily on inbound links alone to determine rankings. At the time, many businesses in Google’s business index didn’t even have websites; some still don’t. Without a website, there’s nothing for other sites around the web to link to. So Google had to develop an alternative ranking algorithm that wasn’t dependent on links.
These Name, Address, and Phone number (NAP) mentions online are what we know as citations. Your NAP is your digital thumbprint — it’s how Google knows that a website mentions your business as opposed to someone else’s. The more times Google sees your thumbprint on reputable websites, the more confident it is displaying a reputable company in its search results. Remember to be consistent anywhere you expect Google to pick up your thumbprint.
Mixing and matching your NAP makes it much harder for Google to match mentions of your business. Subsequently, giving your business credit through rankings is more challenging. If those mismatches appear in prominent enough sources, they can lead to duplicate listings. This is a headache that no business wants to develop.
NAP consistency is critical between your website and Google My Business. Yoast Local SEO makes this two-way consistency easy.
Where to get citations
Unless you’re blatantly spamming, there isn’t a bad website on which to acquire a citation. But as with inbound links, specific citations are more valuable than others. Let’s take a look at the most valuable citation types below.
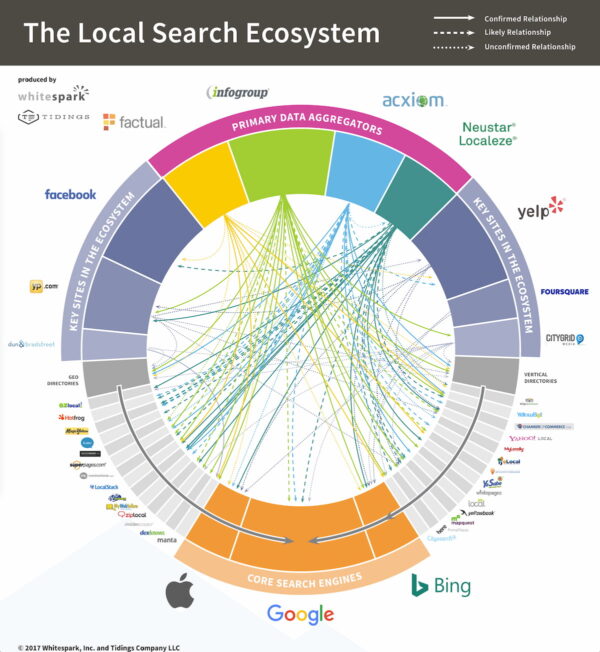
Data aggregators
Google has licensed existing databases in most countries to build its local business index rather than starting from scratch. In many cases, the licensors are the most prominent traditional yellow pages companies in each market. See how this local search ecosystem is connected.
Consumer directories
In addition to licensing data, Google searches the internet for local business citations. Citations from authoritative consumer directories (such as Foursquare, Yelp, or YP.com) carry more weight in helping your rankings than those from weak directories you’ve never heard of, like USCity.Net or ABLocal.
Whitespark has put together great resources that uncover the top consumer directories on which you should list your business.
The critical point is that the citation source’s quality matters far more than the number of sources you’re listed. Despite the marketing of certain business listing services touting “dozens” or “hundreds” of directories, the reality is that there are only a handful of cross-industry consumer directories on which you need to be listed.
Industry directories
As with inbound links, citations from industry-relevant websites help build the authority of your business. They also give Google a sense of the keywords for which your business is relevant.
Chances are that U.S.-based businesses can rattle off the important vertical directories in their industry. Sites like Avvo and Findlaw for Lawyers, Houzz and HomeAdvisor for contractors, WeddingWire and TheKnot for photographers, etc. These are the directories that regularly rank for the keywords that you want to rank for.
Businesses with an optimized thumbprint on these directories stand a better chance of ranking in Google for industry terms than businesses with a messy or missing thumbprint. Whitespark has put together a list of the top industry directories.
Local directories
Citations from local directories also increase the authority and credibility of listed businesses. As we mentioned, the member directories of your local chamber of commerce and neighborhood business association are great places to start. There may also be business listing websites that are popular with residents. Seek listings on similar sites in your business’s towns and cities.
On building citations online
It’s essential to be represented as cleanly and entirely as possible in as many online places as possible. However, it’s necessary to weigh the benefit of citations against their cost – whether in time or money.
Be where your customers expect you to be. If you run a deli, and every other deli in your city is on Yelp, you probably need to be on Yelp, too. If you’re a home decorator, and every other home decorator in your region is on Thumbtack, you probably need to be on Thumbtack, too.
Being where your customers expect you also means you’ll be where Google expects you to be. Citations beyond these prominent websites provide diminishing returns, so be wary of that fact as you evaluate signing up for new products or services.
Citations in the future
The importance of citations has gradually decreased over the years. Citations are a rudimentary ranking factor in an increasingly sophisticated local algorithm. Because they’re relatively easy to build, most successful small businesses will already have a strong citation profile.
In other words, citations have table stakes in the Local SEO poker game. You need a strong citation profile to compete. But if your business already has a strong profile, it’s unlikely that building a few more citations will move the needle much on your rankings.
Reviews: more important by the day
Although they weren’t part of the initial release of Google Maps, reviews have been a fixture on Google’s local properties for over fifteen years. The reason is obvious: consumers love reviews.
A considerable amount of consumers use reviews to evaluate local businesses. Many of them trust reviews just as much as a personal recommendation! So it’s no wonder that Google features them so prominently.
It stands to reason that if consumers love reviews so much, Google’s ranking algorithm does too. Businesses with robust review profiles on Google – and beyond – tend to be rewarded with higher rankings.
Reviews create a virtuous cycle. More reviews lead to better visibility, which leads to more customers, which results in more reviews. Simply gathering and encouraging customer reviews is one of the most sustainable marketing techniques your business can engage in.
How Google evaluates reviews
Only the engineers know, but local search experts have theorized that Google primarily evaluates reviews across the attributes below for years. But what are they looking for?
Volume
Google designed its entire local algorithm to represent the offline world online in the most accurate way possible. In Google’s ideal world, popular businesses rank near the top of search results. Less popular businesses rank further down. Reviews are one of the easiest ways for Google to assess popularity.
All other factors being equal, popular businesses tend to serve more customers than less popular ones. But remember, Google can only “see” what’s represented online. So if your customers leave reviews of your business at a higher rate than your competitors’ customers, your business will appear more popular and stand a good chance at outranking the competition.
Content
The area in which Google’s algorithm has arguably improved the most over the past years is semantic analysis and neural language processing. One of the earliest datasets on which Google trained its semantic algorithm was local business reviews.
So not only is Google looking at the number of reviews when assessing the popularity of local businesses, it’s looking at what people are saying about local businesses in those reviews. For example, doctors whose patients frequently mention a particular treatment in their reviews will likely rank better in searches for that treatment. Contractors whose customers say the kind of projects they execute, such as “kitchen remodel,” are likely to rank better for searches for those kinds of projects.
The content of your customers’ reviews isn’t necessarily something you can control. But prompting your customers to think about particular questions as they write their reviews (“What service did we perform for you?” e.g.) can help improve the effectiveness of those reviews concerning your rankings.
Diversity
A common misconception is that Google does not use third-party reviews to rank local results. This could not be further from the truth. In some cases, reviews on third-party sites can improve your rankings even more than comparable reviews left directly at Google. It’s not only a best practice but also essential to earn reviews from your customers on some sites beyond Google.
Star / numerical rating
Generally speaking, Google’s algorithm seems to value volume and sentiment much more strongly than the star rating that customers leave for a business. With nearly 75% of reviews being three stars or above (even on Yelp!), it’s not particularly useful for Google to split hairs between a 4.2 and a 4.4-star business, for example.
Where rating may play a larger role is in consumer choice. According to BrightLocal, a majority of consumers see the rating as the most important review factor in choosing a business.
The reviewer
Google’s review spam filter leaves much to be desired. There is, however, some evidence to suggest that the reviewer’s account may positively influence how much weight his or her review carries. In much the same way Yelp Elite reviews carry extra weight in Yelp’s algorithm, reviews from members of the Local Guides Program likely carry extra weight in Google’s.
Velocity
The velocity or frequency customers leave reviews may also impact a business’s rankings. Older reviews might be seen as no longer relevant. While Google’s “review expiration date” is longer than three months, especially in less-frequently-reviewed industries like DUI law or addiction treatment, businesses with a steady stream of new reviews will likely outrank those with a stale review profile.
Where to get reviews
Don’t focus your review acquisition efforts solely on Google. Reviews on prominent sites like Yelp have been proven to single-handedly increase rankings for businesses in smaller markets with limited competition.
As with citations, you want to have reviews on the sites where Google expects popular businesses to have reviews. The only difference between the sites where you should acquire citations vs. the sites where you should acquire reviews is that data aggregators don’t offer reviews as a feature.
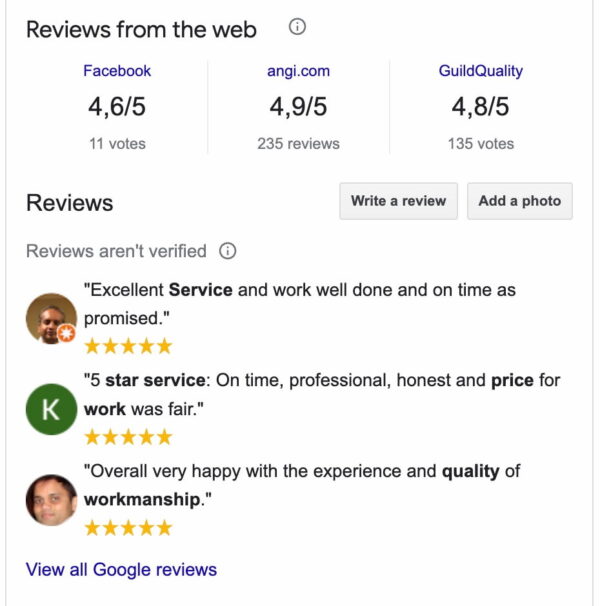
Consumer directories
You should do your best to acquire customer reviews on Facebook and Yelp. These two platforms are used to research local businesses by millions of consumers monthly. Yelp syndicates its reviews to Apple Maps. This way, even more consumers will read them. And, of course, Facebook is Facebook — although slowly losing favor, it’s still an important platform for many.
Industry-specific and local reviews
Beyond these two giants, you should look at the sites that show up in Knowledge Panels for your competitors. Also, look at other high-ranking businesses similar to yours in different geographic markets.
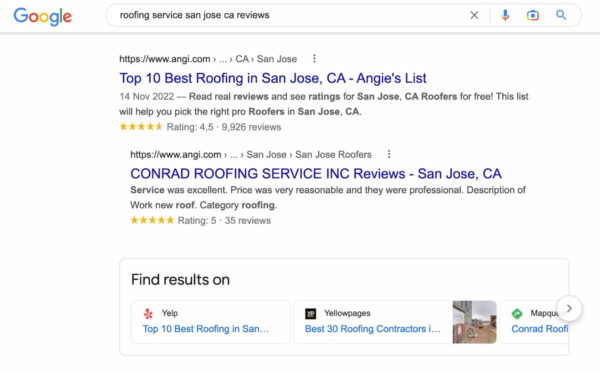
Sites like the ones listed in the ‘Reviews from the web’ sections of Knowledge Panels likely have direct relationships with Google to feed them reviews. Also, take a look at the review sites that show up for searches matching the pattern:
[your keyword] [your city] [reviews]
Note the review sites that appear in the top 20 (or so) organic results. Pay close attention to the ones with gold stars in the organic results.
How to get reviews
Have an intentional review acquisition process in place because it’s an essential element of success for your local search strategy.
Knowing the importance of customer reviews, you might be tempted to blast all your customers at once, asking them to leave reviews. Or worse, you might consider buying your way to the top with fake reviews from Fiverr or similar sites. These techniques might lead to success in the short term but dramatic pain in the long term. Google and other review platforms are getting better at cracking down on this behavior. This is pretty trivial to spot algorithmically.
Instead, a steady drip of reviews will lead to sustained long-term success. Depending on your industry, this could be a handful per month or a handful per week.
Getting Yelp reviews
Getting Yelp reviews can be challenging, thanks to Yelp’s overaggressive review filter and historically asinine policy on review solicitation. You’re not supposed to ask people to leave reviews on Yelp, so your best bet is to try and get these organically.
Under no circumstances should you offer an incentive to leave a review on Yelp — or any other platform, for that matter. This is a violation that will get you blocklisted. If the incentive is not disclosed, it may violate United States FTC guidelines or similar laws in other countries.
Responding to reviews
No matter how great your business is, you’ll get a negative review at some point. Many sites, including Google and Yelp, allow you to respond to that bad review as a business owner. The critical thing to remember is that the real audience for that response is not this particular customer but the dozens or hundreds of prospective customers who read your response, evaluate your empathy for the reviewer and attempt to resolve the complaint.
What’s next for reviews
The reality is that reviews are a far more democratic ranking signal than inbound links or even citations. They more accurately reflect the popularity of a business than either of these prominent local ranking factors.
Half of the consumers asked by a local business for a review have left one. This is an exponentially higher fraction than the number of consumers who operate websites, let alone have given a local business a link from those websites!
While Google has a long road ahead in fighting spam, it will shut down the most egregious spammers within the next few years. And as long as consumers continue to make decisions at least partially based on reviews, they’ll be a fixture in local search results (and rankings) for years to come.
Behavioral signals
As one of the most pervasive companies, Google has as much data about our behavior as any company in human history. Only Google has a full picture of user behavior, so it’s the blackest of Google’s many algorithmic black boxes. But experts in the Local Search Ranking Factors survey have pegged these signals in the top eight most crucial factors and competitive difference-makers.
But Google’s longstanding mission in local search has been to reflect the real world as accurately as possible online. A reflection based on real-world human beings will be far more accurate than one based on data from digital-world webpages and robots. It stands to reason that as Google can gather more real-world behavioral data, it will grow in algorithmic importance for rankings.
Let’s look at some of the behavioral data that Google is likely using to inform local rankings and search, from most basic to most advanced.
Location of searcher
Google has always been very good at detecting location on mobile phones. Now, they are scarily good even for desktop searches. And while it’s hard to describe something as sophisticated as detecting a user’s location as “basic,” the algorithmic outcome of that location is relatively straightforward.
The distance of a business from the location where a search is performed influences how well it ranks for those searches. All other factors being equal, the closer the company is to the point of search, the higher it will rank.
Beyond numeric rankings, the radius of businesses Google considers proximally relevant varies by category. High-frequency brick-and-mortar businesses like coffee shops have a tighter radius of relevance. Low-frequency or service-area businesses like golf courses or roofing companies have a wider radius.
Suppose your business lies outside this relevancy radius from the search locations of large groups of your customers. In that case, you will have a tough time attracting those customers via Google.
Branded search volume
In a way, branded searches are a kind of citation. If corroborated by information in Google’s business database, they’re an expression of interest in that business — if not an out-and-out endorsement. While branded searches are a fundamental indicator of the awareness or popularity of a company, most internet users perform these regularly, making them one of the most democratic ranking signals.
Beyond just the number of times a brand name is searched (and searched by people in a given geographic area), the context of those brand names is also important. Adjacent keywords used in those searches that rank for future unbranded searches for those keywords.
Generally, branded searches favor established businesses over new ones and companies that take a holistic marketing approach, so including offline. They’re one of Google’s best heuristics for word-of-mouth as it tries to build its reflection of the offline world.
Click Through Rate
There’s an endless discussion around Click Through Rate (CTR) as a ranking factor in organic search. The theory is that the more people click on your listing or website in a given search result, the more times it will show up for similar searches in the future. CTR is one step up from a branded search. CTR indicates, if not endorsement, that the searcher thinks the destination listing or website will be relevant to her query.
Google has never shared information about the inner workings of this ranking factor — and explicitly obfuscated its usage. But SEO practitioners suspect there’s a mechanism involving CTR relative to the position on the page. After all, the top results will always get the lion’s share of clicks.
You can improve your organic CTR with more compelling titles and meta descriptions on your web pages. Your Google Business Profile listings have fewer options, but a superior review profile — both star rating and volume — will help you stand out from the competition and earn more than your share of clicks.
Personalization
Much of the account infrastructure underlying Google’s products (Search, Gmail, Maps, YouTube, etc.) has been unified. As a result, we’re all perpetually logged in to the same account on every device. Some devices, like Android phones and Google Home, require users to log into their Google accounts before using them.
As a tracking and data-gathering platform, Google has been a smashing success. It’s now trivial for Google to track us from desktop to mobile to tablet, from Gmail to Maps to YouTube to Search, and back again. Our behavior in each product and on each device informs what we see in different effects on different devices.
At a strategic level, you should do everything you can to engage your customers with reasons to return to your website, engage with your email newsletter, and share your business with their friends and family via email and messaging. Google is probably monitoring all of those visits and shares. It may use them to inform future search results for those customers, friends, and family, even if they don’t convert on their initial visit.
Knowledge Panel interactions
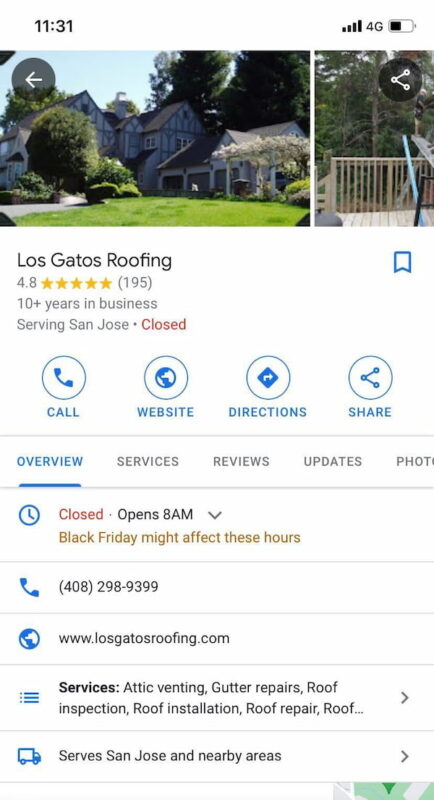
As Google displays more and more Knowledge Panel results, the percentage of clickthroughs to webpages has dropped. But that doesn’t mean searchers are no longer clicking at all. Increasingly, clicks are happening within Knowledge Panels.
These Knowledge Panel click-throughs are far more substantial endorsements of a business’s relevance for a given query than a website visit. They’re a direct indication of a desire to transact with the company.
Phone calls
Google has offered mobile click-to-call functionality since January 2010. Even as early as February 2014, 40% of searchers had used it.
Driving directions
Where a phone call indicates a desire to learn more about a business, a request for driving directions is an even stronger indicator that a searcher intends to visit that business. The strongest of all purely digital signals that a business is relevant for a particular query.
Bookings (where available)
Google has long offered users the ability to make bookings with hotels and restaurants directly from the Knowledge Panel through partnerships with Expedia, OpenTable, and others. There are many other options, and businesses can now add their own booking buttons with the Maps Booking API feature.
By offering this in-SERP interactivity with a business directly through Knowledge Panels, Google reduces the number of clicks to business websites and can collect more data about how searchers view a business. However, this data influences rankings, as with most behavioral signals, only Google knows how much.
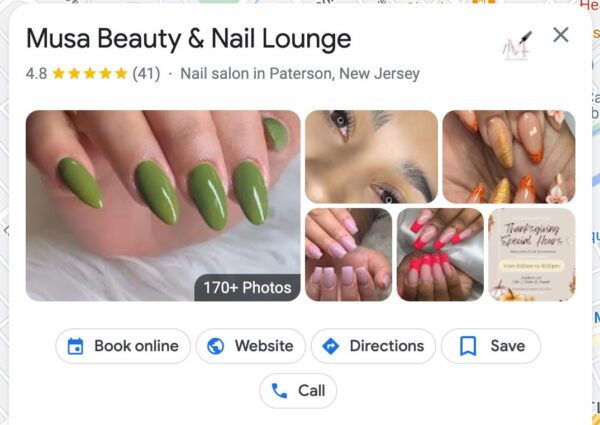
In-store visits
It’s reasonable to expect Google to track our on-SERP and click behavior online. It has a near-complete picture of our offline behavior through its perpetual location-tracking of Android and iOS users with the Google Maps app installed. We see the outcome of this tracking in the Popular Times section of many businesses’ Knowledge Panels.
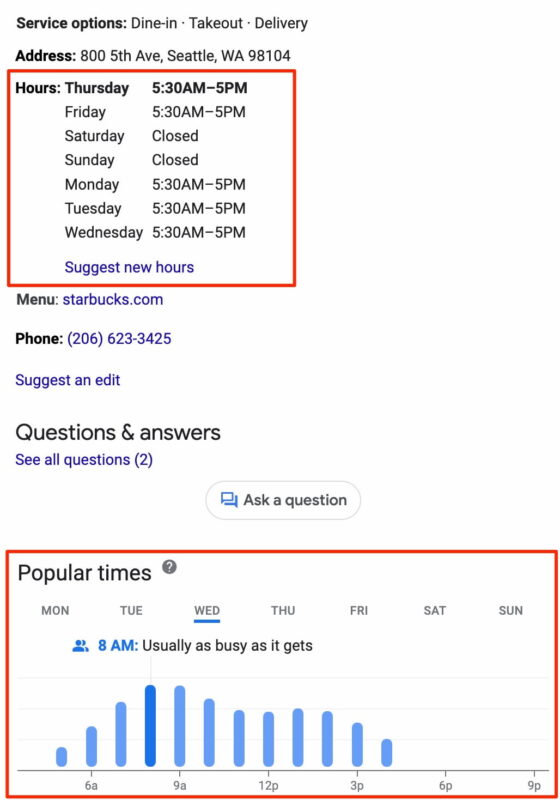
Google aggregates location data from any person it can — whether they’ve searched for a business or not — and puts that data front-and-center on that business’s Knowledge Panel. It even tracks how long people stay at a given company and whether the business is busier or less busy than usual.
This complete offline tracking helps Google offer its advertisers a “closed loop” of data on whether online ads lead to offline visits. To think that Google isn’t using this same closed loop of data for its local algorithm defies belief.
Regardless of whether knowing a business’s popularity before you visit is an acceptable tradeoff for your privacy, offline visits are the ranking signals that help Google identify local popularity and relevance most accurately — and they can’t be optimized.
Offline transactions
It took a while, but Google succeeded in the mobile payment space. Google Pay is now only second to Apple Pay and is closely followed by Samsung Pay. It’s hard to ignore data from tens of millions of consumers. Particularly in industries with frequent purchases like supermarkets, coffee shops, and gas stations, the volume of Google Pay transactions could be a reasonable indicator of the offline popularity of a business.
But Google is not only looking at mobile payments — it’s now looking at all costs. In 2017, Google partnered with credit card companies to track 70% of all consumer purchases. And in 2019, it was found that Google also keeps track of your other purchases by checking your receipts in Gmail. Google is increasingly looking to bridge the gap between the real world and the online world — especially in the commerce space –, so we can expect more on this front.
Social media is nice to have
Generally speaking, social media is not an essential part of local SEO. You should do the basics well. Primarily, “the basics” involve optimizing your social media profiles instead of your social media activity.
Social media basics for your local search strategy
At a minimum, every local business should claim a business profile on Facebook, Twitter, LinkedIn, Pinterest, and Instagram, even if you don’t plan to use some or all of those profiles.
Customers may look for you on those sites, and you don’t want them to come up empty, or worse: discover another business with a similar name and think it’s you. And you never know when you might decide to engage with customers on those social platforms – in which case, it’ll be nice to have an existing profile as a jumping-off point.
Social profiles offer some of the easiest inbound links and citations you can acquire, and it makes sense to utilize all relevant fields that major social media platforms offer you.
Setting up the social media profiles
At a minimum, use a high-quality logo or, if more appropriate, a personal photo. Pick a high-resolution photo or graphic representation of your business that you can use as a cover image. Hubspot produced this handy guide of the sizes you’ll need for each social platform.
Because each social profile can (and should) act as a citation, you’ll want to maintain a consistent business name across all platforms. This helps Google (and customers) associate these profiles with you.
Where possible, add your location information to your profile, even if it’s a city and state. This helps Google make that connection even more strongly.
If you don’t plan to use one or more of these profiles actively, pin a post to the top of that profile. That way, you can let customers know where they can find you. It doesn’t matter if that’s your website, email newsletter, or a different social channel you manage actively.
Social media and local search in the long tail
Except for Twitter, with whom it has a direct contractual relationship, Google has difficulty getting visibility into what’s happening on social platforms. So “being active” on social media doesn’t help your local search visibility. And even if you’re wildly popular on social media, it’s unlikely that popularity will translate directly into higher local search rankings.
One way it might translate is if your social profile is frequently linked to other websites due to your popularity. The link you’ve added from your profile to your website then passes additional authority to your website. But that’s a fraction of a fractional increase in authority. Not one that’s worth getting hung up on.
You should focus your social media efforts on engaging your customers with exciting content, promotions (if relevant), and polls and conversations that will increase their affinity for your brand. You can promote your website to a degree, but generally speaking, improvements in your local rankings will come from other factors.
Social media is for conversations
It’s far more productive to treat social media as an engagement channel rather than a means to rank better. Making yourself available to your customers and responsive to their questions on the platforms above — as well as the locally-focused NextDoor — helps create the positive association for your brand that social media is best designed for. Utilize your social media channels for brand awareness, customer engagement, and loyalty, not rankings.
Local SEO conclusion
Local search has become a multi-faceted paradox in the last couple of years. While the algorithm has evolved to reward real-world behavior, the SERP interface rewards more technical tactics like Schema markup and rich snippets.
And while the sophistication of Google’s algorithm and the number of local businesses paying attention to local SEO makes it more challenging than ever to rank, the payoff may be lower as fewer businesses win organic real estate above the fold.
But Google isn’t going away anytime soon. Organic search results will continue to be an effective customer acquisition channel far into the future. Regardless of how Google changes over time, the techniques we’ve laid out in this local search strategy guide should help position your business effectively for the next innovations!

As the title of the article mentioned it’s the ultimate guide, it contains detailed information everyone needs to know in order to grow their business, I’m searching for tools that I can use for an email campaign I’m working on and I was advised to use Minelead email finder to build my list and for creating the campaign, I wonder if you tried it before and what are your thoughts about it.
Hi there, Karima! It’s great that you’re working on growing your business! Unfortunately, we have no experience with the tool you mentioned. Sorry about that. Lots of luck with your marketing and SEO :)
The TOC links are messed up?
Thank you for letting us know, they’re fixed now! :)